Adsorption of chlorine on Ru(0001)—A combined density functional theory and quantitative low energy electron diffraction study
Published:
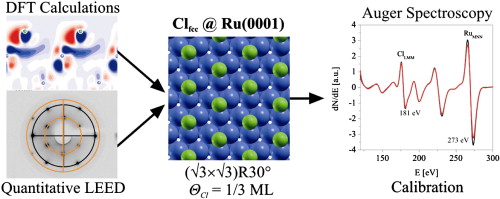
Highlights
- p(√3 × √3)R30°-Cl phase, ΘCl = 1/3 ML in fcc sites identified to be the most stable adsorbate structure on Ru(0001).
- Structural parameters of this phase determined by quantitative LEED (rP = 0.19) and DFT calculations.
- p(√3 × √3)R30°-Cl island formation indicated by LEED at low Cl coverage.
- Complex compressed overlayer structures form at Cl coverage > 1/3 ML.
- Structure can be used for Auger electron intensity calibration.
Abstract
Chlorine adsorption on Ru(0001) surface has been studied by a combined density functional theory (DFT) and quantitative low energy electron diffraction (LEED) approach. The (√3 × √3)R30°-Cl phase with ΘCl = 1/3 ML and chlorine sitting in fcc sites has been identified by DFT calculations as the most stable chlorine adsorbate structure on Ru(0001) with an adsorption energy of − 220 kJ/mol. The atomic geometry of (√3 × √3)R30°-Cl was determined by quantitative LEED. The achieved agreement between experimental and simulated LEED data is quantified by a Pendry factor of rP = 0.19 for a fcc adsorption site with a Cl-Ru bond length of 2.52 Å. At chlorine coverages beyond 1/3 ML LEED reveals diffuse diffraction rings, indicating a continuous compression of the hexagonal Cl overlayer with a preferred average Cl–Cl distance of 4.7 Å in the (√3 × √3)R30°-Cl, ΘCl = 1/3 ML phase towards 3.9 Å at saturation coverage of 0.48 ML.
Links
J.P. Hofmann, S.F. Rohrlack, F. Hess, J.C. Goritzka, P.P.T. Krause, A.P. Seitsonen, W. Moritz, H. Over. Surf. Sci. 606 (2012) 297-304. 10.1016/j.susc.2011.10.010
