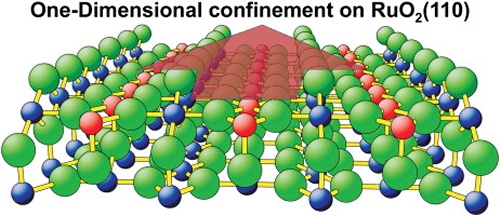
One-dimensional confinement in heterogeneous catalysis: Trapped oxygen on RuO2(110) model catalysts
Published:
Featured in a perspective by W.F. Schneider: Configurational control in catalysis: Perspective on Hess et al., One-dimensional confinement in heterogeneous catalysis: Trapped oxygen on RuO2(110) model catalysts
Abstract
With temperature programmed reaction (TPR) experiments and kinetic Monte Carlo (kMC) simulations of coadsorbed oxygen and HCl on the RuO2(110) surface we studied the thermal stabilization of dissociatively adsorbed oxygen. Due to one-dimensional confinement single surface O atoms can be trapped by surface chlorine atoms so that surface oxygen is not able to desorb from the RuO2(110) surface at the expected temperature of 420 K. Trapped oxygen needs desorption temperatures as high as 700 K where it recombines with bridging O from RuO2(110) to form O2. Kinetic modeling of catalytic reactions with dimensional confinement of their reaction intermediates on the catalyst’s surface requires the application of kinetic Monte Carlo simulations which are beyond the mean field approach.
Links
F. Hess, P.P.T. Krause, S.F. Rohrlack, J.P. Hofmann, H. Over. Surf. Sci. 606 (2012) L69-L73. 10.1016/j.susc.2012.04.019
