Probing the Activity of Different Oxygen Species in the CO Oxidation over RuO2(110) by Combining Transient Reflection–Absorption Infrared Spectroscopy with Kinetic Monte Carlo Simulations
Published:
Abstract
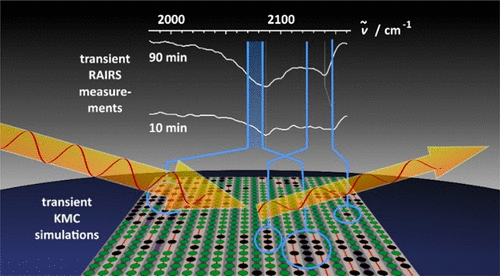
Transient spectroscopic surface-chemistry experiments in combination with spatially resolved kinetic Monte Carlo (KMC) simulations offer great potential to gain a wealth of molecular information on the kinetics of catalytic surface reactions as exemplified by the CO oxidation reaction over RuO2(110). This approach surpasses the common problem that in the steady-state reactions, the prevailing species detectable by in operando surface-sensitive spectroscopy are frequently spectator species, thereby obscuring the reactive surface species. Our experiment is sensitive to the relative activity of different oxygen species by saturating the surface with loosely bound oxygen, leaving only single vacancies where CO can adsorb and recombine with oxygen. With in situ reflection–absorption infrared spectroscopy (RAIRS) in combination with ab initio based KMC simulations, we follow the time evolution toward steady state (transient experiment). In this way, we are able to resolve a long-standing controversy about the active oxygen species in the CO oxidation over RuO2(110), evidencing that both surface O species (Obr and Oot) are equally active, although their adsorption energies differ by more than 150 kJ/mol.
Links
F. Hess, C. Sack, D. Langsdorf, H. Over. ACS Catal. 7 (2017) 8420-8428. 10.1021/acscatal.7b02838
