The stabilizing effect of water and high reaction temperatures on the CeO2-catalyst in the harsh HCl oxidation reaction
Published:
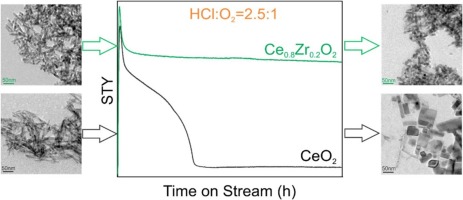
Highlights
- Deacon process: Deactivation of CeO2 catalyst proceeds through bulk chlorination.
- Reaction temperatures above 390 °C and little water (1%) in the reaction feed stabilize CeO2 against bulk-chlorination.
- Chlorination of the CeO2 catalyst start at the inlet and propagates through the catalyst bed.
Abstract
We studied the stability of CeO2 nano-cubes with preferentially (100)-oriented facets in the HCl oxidation reaction (Deacon process) for various reaction temperatures and the addition of small concentrations of water in the gas feed. For a reaction mixture HCl:O2 = 1:2 we find that CeO2 is substantially chlorinated below 380 °C, revealing a low catalytic activity. At 390 °C both the activity and the chlorination degree change abruptly: activity becomes high and chlorination is not detectable by XRD. The experimental results are rationalized by a kinetic model which allows us to study catalyst chlorination as a function of temperature and gas feed composition. The model predicts that chlorination sets in at the inlet of the catalyst bed and propagates then slowly along the catalyst bed towards the reactor outlet to fully chlorinate the CeO2 catalyst bed. This process has been confirmed by a dedicated experiment employing two separate catalyst layers, where only the first layer is shown to be chlorinated while exposed to the Deacon gas feed. Our model attributes the excessive chlorination at the reactor inlet to the absence of H2O in the gas feed, as the formation of H2O by the reaction of HCl with CeO2 is the prevailing driving force for catalyst chlorination. When running the Deacon process at 375 °C, the chlorination of CeO2 nano-cubes is efficiently suppressed by the addition of 1% water to the reaction mixture. This extrinsic stabilization of oxide catalysts towards chlorination by water is a general concept, which may enable the identification of new oxide materials that have previously been ruled out as Deacon catalysts due to their low stability under reaction conditions.
Links
C. Li, F. Hess, I. Djerdj, G. Chai, Y. Sun, Y. Guo, B.M. Smarsly, H. Over. J. Catal. 357 (2018) 257-262. 10.1016/j.jcat.2017.11.019
