Electrochemical polarization dependence of the elastic and electrostatic driving forces to aliovalent dopant segregation on LaMnO3
Published:
Abstract
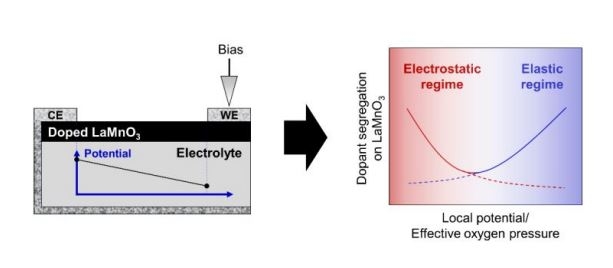
Maintaining a stable surface structure and composition is important for retaining long-term reactivity to oxygen exchange reactions on perovskite oxides in energy conversion applications. A common detrimental surface evolution is the segregation of aliovalent dopant cations, for example Sr2+ in La1-xSrxMnO3 (LSM). This process is known to be activated at elevated temperatures. Here we focus on resolving the effect of the oxygen chemical potential, which varies over a wide range in electrochemical and thermochemical energy conversion reactions. We employ electrochemical polarization to tune the oxygen chemical potential over many orders of magnitude, quantify the resulting segregation by X-ray photoelectron spectroscopy, and employ ab-initio thermodynamics calculations to rationalize our findings. Ca-, Sr-, and Ba-doped LaMnO3 is selected as a model system, where the dopants have the same charge but different ionic sizes. Altering the effective oxygen chemical potential alters the oxygen non-stoichiometry in the electrode, which then influences the mechanisms underlying the segregation of aliovalent dopants. These mechanisms are (i) the formation of charged defects that couples to the electrostatic energy of the dopant in the perovskite lattice, and (ii) the elastic energy of the dopant due to cation size mismatch, which also promotes the reaction of the dopant with O2 from the environment. The present study probes the balance of these two contributions by monitoring the segregation of dopants under polarization, spanning a range from -0.8 V to +0.8 V, equivalent to a change in the effective oxygen pressure of 31 orders of magnitude. The reducing conditions achieved via cathodic polarization promote a high concentration of surface oxygen vacancies, which electrostatically attract negatively charged dopant cations. Under oxidizing conditions (anodic bias), reactions of the dopant with O2 from the environment leads to the formation of secondary dopant oxide and peroxide phases, and the reactivity to form these products depends on the dopant cation size mismatch with respect to the host cation La. This results in the most enrichment for Ba and the least for Ca under anodic polarization conditions. We found progressive enhancement of Sr and Ba segregation with increasing bias voltage for both polarities. This observation is interpreted as a transition between the electrostatically and elastically dominated segregation regimes. The transition between these regimes shifts to a lower potential with increasing cation size, as seen in our experiments and predicted by our ab-initio thermodynamics calculations. In contrast, Ca segregation exclusively responds to changes in electrostatic energy due to the small mismatch in size between Ca and the host cation, La. The present study provides quantitative insights into how the elastic energy (manifested by lattice parameter and cation size) and the electrostatic energy (manifested by charged defect concentration and distribution) determine the extent of segregation for a given polarization and atmosphere relevant to the operating conditions of perovskite oxide materials in energy conversion applications.
Links
D. Kim, R. Bliem, F. Hess, J.-J. Gallet, and B. Yildiz. J. Am. Chem. Soc. 142 (2020) 3548–3563. 10.1021/jacs.9b13040
