Reactivation of CeO2-based Catalysts in the HCl Oxidation Reaction: In situ Quantification of the Degree of Chlorination and Kinetic Modeling
Published:
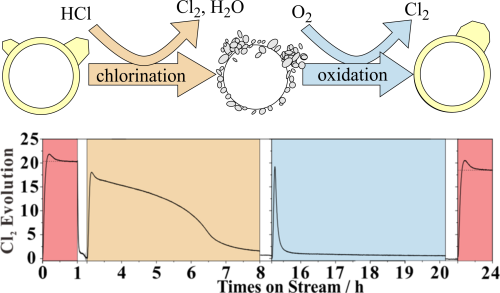
Abstract
Deactivation of CeO2-based catalysts in the HCl oxidation reaction proceeds via selective bulk chlorination of the active CeO2 component to form CeCl3×nH2O. We study the reactivation of two bulk-chlorinated CeO2-based Deacon catalysts by oxygen treatment at 430°C, namely pure CeO2 and 20 mol% of CeO2 supported on preformed ZrO2 particles (20CeO2@ZrO2), with a dedicated experiment. In the flow reactor setup we determine in situ the degree of chlorination of the catalyst by quantifying downstream with in-situ UV-Vis spectroscopy the total amount of chlorine in the catalyst that is exchanged by reoxidation at 430°C. The activity of deactivated 20CeO2@ZrO2 can be fully restored by oxygen exposure at 430°C, while that of pure CeO2 declines steadily. Since the UV-Vis analytics is fast and sensitive, we can follow the kinetics of reoxidation. To rationalize the observed kinetics, we develop a modified Johnson-Mehl-Avrami-Kolmogorov (JMAK) model based on a nucleation-and-growth approach for the reoxidation of the catalyst starting from the chlorinated phase. The fast reoxidation kinetics of chlorinated 20CeO2@ZrO2 is traced to a fast nucleation rate.
Links
Y.Sun, F. Hess, I. Djerdj, Z. Wang, T. Weber, Y.Guo, B.M. Smarsly, and H. Over. ChemCatChem 12 (2020) 5511. 10.1002/cctc.202000907
