Is There a Stable Deacon Catalyst? Computational Screening Approach for the Stability of Oxide Catalysts under Harsh Conditions
Published:
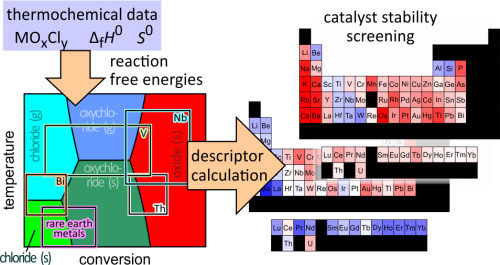
Abstract
Under harsh reaction conditions, lack of catalyst stability can impede the large-scale implementation of a technical process. Current computational screening approaches address catalyst activity or selectivity, but screening models that predict catalyst stability under operating conditions are still lacking. Herein, a screening model is presented that can predict catalyst stability under harsh reaction conditions based on the thermodynamic data of bulk phases while accounting for variations in the operating conditions (temperature, gas feed composition, and conversion). It is applied to oxide catalysts for the oxidation of HCl (Deacon reaction), where catalysts can chlorinate irreversibly or leach from the catalyst bed in the form of volatile chlorides and oxychlorides, resulting in loss of catalyst activity. Two numerical descriptors are computed from the thermodynamic data, ranking the oxides according to their stability against chlorination and vaporization, which enables large-scale screening. The underlying computations provide detailed insights on the possible reactions of the catalyst with the gas stream in the form of reaction free energy diagrams. This allows one to identify where the reactor degradation is the most severe and improve the catalyst stability by addressing these stability bottlenecks. For the Deacon reaction, the oxides and chlorides of 66 elements are examined. The two descriptors are empirically found to be largely complementary, which means that most oxides are prone to either chlorination or volatility, with a few exceptions. It is found that both chlorination and volatility are most severe close to the reactor inlet, which suggests that a highly stable catalyst material must be employed at the reactor inlet. The late rare-earth oxychlorides, as well as Nb2O5 and Ta2O5, are identified as promising candidates with high stability in both categories at the reactor inlet. The developed model can be applied to catalytic processes where phase transformations of the catalyst material under operating conditions are the major cause of catalyst deactivation and are in principle applicable to any process that runs at sufficiently high temperatures.
Links
F. Hess. ACS Catal. 12 (2022) 497–511. 10.1021/acscatal.1c04487
